Home
XYNTHA SOLOFUSE
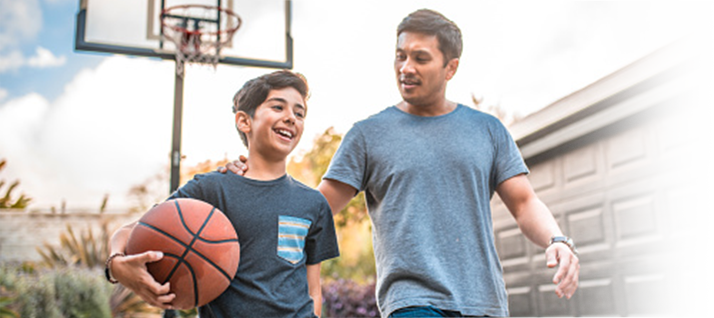

Proven bleed control
and protection
Demonstrated across clinical trials of previously treated patients (PTPs).

All-in-one reconstitution
Be prepared to bring it—preparation with all-in-one reconstitution in a travel-ready kit.
 Not actual patients.
Not actual patients.

Free trial offer*
Whether it is your first time on XYNTHA or if you are switching to prophy, you may be eligible to receive a 1-month trial supply of up to 20,000 IU at no cost.
*Terms and conditions apply.


Resources and support
More than just factor—take a look at some of the resources and support Pfizer Hemophilia has to offer.
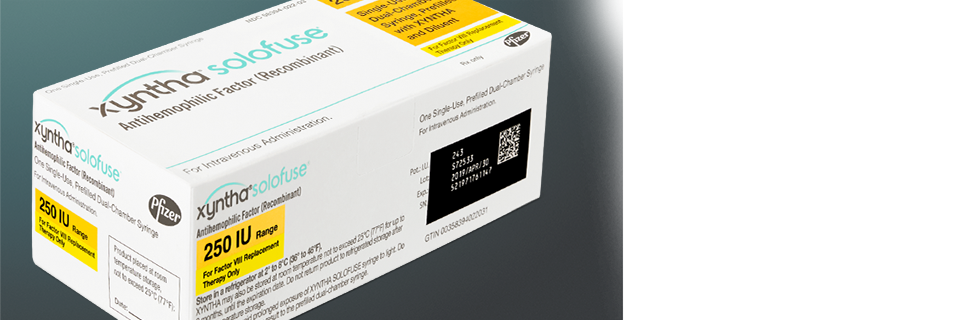

Environmentally friendly
travel-ready kit
The cardboard travel-ready kit still comes compact and loaded with everything you need to infuse on the go.


